43 open-label study disadvantages
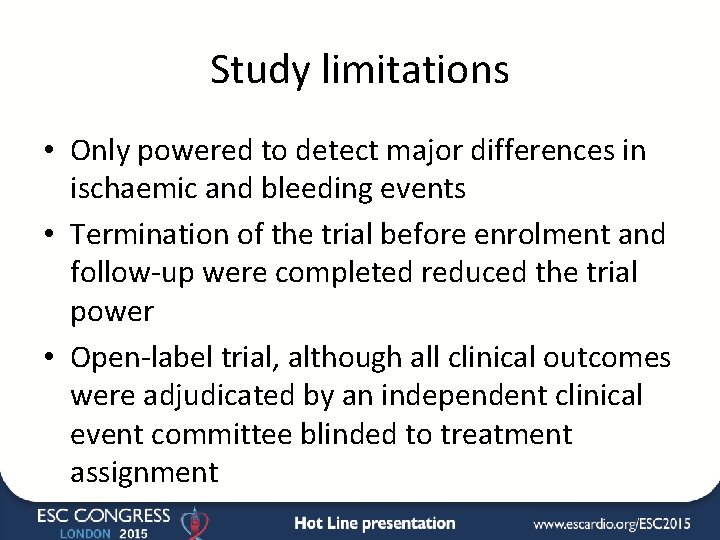
Statistical controversies in clinical research: limitations of open ... Compared with double-blind trials, the use of an open-label design may lead to exaggeration of the estimation of the risk of bleeding, venous thrombotic events and arterial thrombotic events with antiangiogenic treatment by 68%, 53% and 65%, respectively. Introduction What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,...
Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [6-10]. Blinded outcome assessment is often achieved by using an independent clinician who directly examines the patient, or by an independent adjudication committee.

Open-label study disadvantages
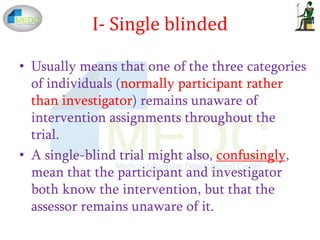
Open-label trial - Wikipedia An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group ), with all participants receiving the same treatment. [4] Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. What is 'Blinding' in Research? What are Its Types? Triple blinded studies also lengthen blinding to the data specialists. In triple blinding, the study participant, the data investigator or data collector and the data analyzer- all are blinded. Only the Principle Investigator of the research might know about the trial- may it be treatment, drugs or so on. 4. Unblinded or open-label:
Open-label study disadvantages. (PDF) What is an open label trial? - ResearchGate this was an open label trial, the participants, investigators, and all peripheral staff were not blinded to the treatment allocation—that is, they were aware which treatment the participants had... Open-Label Extension Studies | SpringerLink The ethical concern that informed consent cannot be given is clear: response while receiving placebo or lack of response to the new medicine by the patient in the 'active' arm of the phase III study would not provide a sensible basis for participation in an open-label extension study. What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. Because this was an open label trial, the participants, investigators, and all peripheral staff were not blinded to the treatment allocation—that is, they were aware which treatment the participants had been allocated to after randomisation ( a is true). Epidemiology and Clinical Research Design, Part 1: Study ... - Europe PMC Open-label study is a type of clinical trial in which the researchers and participants (or parents) know which treatment is being administered. This contrasts with single-blind and double-blind designs. An open-label study may still be randomized. Strengths and limitations of open-label studies:
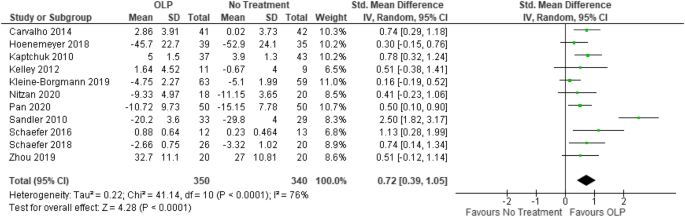
Open Label Study | Semantic Scholar Open Label Study @article{2020OpenLS, title={Open Label Study}, author={}, journal={Definitions}, year={2020} } Published 7 February 2020; Definitions; View via Publisher. qeios.com. Save to Library Save. Create Alert Alert. Cite. Share This Paper. 92 Citations. Highly Influential Citations. 7. Understanding Clinical Trial Terminology: What is an Open Label ... Often, clinical trials use a double-blind approach: study participants and researchers don't know which treatment the patient is receiving. Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random- Effects of open-label placebos in clinical trials: a systematic review ... These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major depression, irritable bowel syndrome and menopausal...
16 Advantages and Disadvantages of a Double-Blind Study - Vittana The FDA even notes that the placebo response is steadily growing in the general population. This disadvantage creates another limitation where the structure of a double-blind study may not provide useful information. 9. Double-blind studies are an expensive effort to pursue. External and internal validity of open label or ... - Wiley Online Library Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias. A Prospective, Open-label, Single-arm Clinical Study the study is a prospective, single-arm, open-label trial, designed to explore the efficacy and safety of Tumor-Treating Fields (TTFields) combined with second-line chemotherapy treatment in recurrent glioblastoma multiforme (GBM) , TTFields is an portable, battery operated device for chronic treatment of patients with recurrent or progressive glioblastoma multiforme (GBM) using alternating ... Open-Label Trial - an overview | ScienceDirect Topics These are uncontrolled, very limited studies that, in the eyes of many researchers, are not formal studies. • Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). They may be informed of this opportunity before or after the ...
Open-label versus double-blind placebo treatment in irritable bowel ... Here, we will briefly review the academic literature on the traditional use of blinded placebos, discuss recent research using open-label placebo (OLP), and describe the methodology and rationale for our current RCT, which was designed to compare open-label and double-blind placebo (DBP) in a sample of participants with irritable bowel syndrome ...
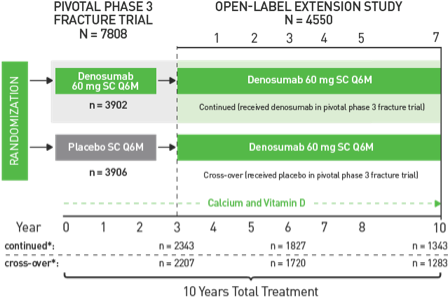
PDF What Are Open-Label Extension Studies For? In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub-
Open-Label Trial | NIH - HIV.gov In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Skip to main content Get the latest public health information from CDC ... Double-Blind Study. Print. Print this term. Download Glossary. English Version PDF(3.13MB) Spanish Version PDF(3.16MB) Connect with us ...
Open-label extension studies: do they provide meaningful information on ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their design.
What is an Open-Label Trial? - Get Essay Writing Help at $10/Page An open-label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. Sometimes the circumstances of a trial make it impossible to conceal the identity of the treatment patients are receiving, and in other cases researchers may have a specific reason for wanting to use the open-label trial format.
Crossover trials: what are they and what are their advantages and ... This term describes the risk that there might be a carry-over from the effect of the previous intervention on to the effect of the next intervention thereby altering results. Therefore, the treatment effect might not only be due to the treatment itself, but also due to interactions between treatment and study-period/sequence.
External and internal validity of open label or double-blind trials in ... In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible.
An Open-label Study Evaluating Ofatumumab Treatment Effectiveness and ... This is a single arm, prospective, multicentre and open-label, 96-week study to evaluate the treatment effectiveness of ofatumumab (OMB) in subjects with relapsing multiple sclerosis (RMS) transitioning from fumarate-based RMS approved therapies, such as dimethyl fumarate (DMF), diroximel fumarate (DRF), and monomethyl fumarate (MMF), or fingolimod due to breakthrough disease activity.
Disadvantages of private label Free Essays | Studymode Private Label. PRIVATE LABEL BRANDS AND THEIR PERCEPTION AMONG INDIAN YOUTH Dr. Ankit Mehrotra Faculty Jaipuria Institute of Management‚ Lucknow Dr. Reeti Agarwal Faculty (Marketing)‚ Jaipuria Institute of Management‚ Lucknow Article No: 180 Year:November 2009 ISSN 0974 - 9497 Volume 3‚ Issue 4/4 Abstract: Retail and real estate are the two booming sectors of India in the present times.
What is 'Blinding' in Research? What are Its Types? Triple blinded studies also lengthen blinding to the data specialists. In triple blinding, the study participant, the data investigator or data collector and the data analyzer- all are blinded. Only the Principle Investigator of the research might know about the trial- may it be treatment, drugs or so on. 4. Unblinded or open-label:
Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial.
Open-label trial - Wikipedia An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group ), with all participants receiving the same treatment. [4]


![PDF) [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with ...](https://i1.rgstatic.net/publication/349228798_177LuLu-PSMA-617_versus_cabazitaxel_in_patients_with_metastatic_castration-resistant_prostate_cancer_TheraP_a_randomised_open-label_phase_2_trial/links/6055b8f7299bf173675532d3/largepreview.png)


















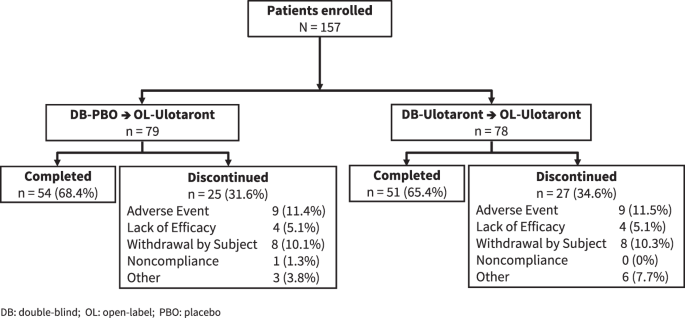




Post a Comment for "43 open-label study disadvantages"