39 open-label study advantages and disadvantages
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to... Pros & Cons of Labeling in Special Education - Study.com The first step in training is to ensure that all teachers understand the concept of labeling. Labeling refers to the process of identifying that a student meets eligibility criteria for special ...
Advantages and disadvantages of clinical trials Possible disadvantages. The new treatment may not be any better than your current treatment. There may be more side effects compared to the standard treatment. Trials may be carried out at a different hospital and involve travel, which can be tiring and take up a lot of time. You may have to go to hospital more often for tests and treatment ...
Open-label study advantages and disadvantages
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,... External and internal validity of open label or double ... - ResearchGate As a consequence, an intense discussion of the advantages and disadvantages of open-label or double-blind trials is currently under way and interpretation of trial results is often focused on this ... Reducing bias in open-label trials where blinded outcome assessment is ... This can be an issue in trials assessing surgical interventions, device trials, or other non-pharmacologic interventions, which are more difficult to blind than traditional drug trials [ 4 ]. Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations.
Open-label study advantages and disadvantages. Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized. 14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Advantages of Randomized Controlled Trials. 1. Randomization prevents the deliberate manipulation of results. A randomized controlled trial works to prevent skewing or the deliberate manipulation of results by researchers or participants. Because each subject gets assigned to a specific group randomly, the removal of choice works to ... Self-Manage Scleroderma | Lesson Patients have a chance to help others and improve patient care. Some disadvantages might be: New treatments or interventions under study are not always better than, or even as good as, standard care. Even if a new treatment has benefits, it may not work for everyone. Health insurance and managed care providers don't always cover clinical trials. Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients ( N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of … 16 Advantages and Disadvantages of a Double-Blind Study The FDA even notes that the placebo response is steadily growing in the general population. This disadvantage creates another limitation where the structure of a double-blind study may not provide useful information. 9. Double-blind studies are an expensive effort to pursue. Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments. External and internal validity of open label or double‐blind trials in ... In these trials, open‐label or double‐blind double‐dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
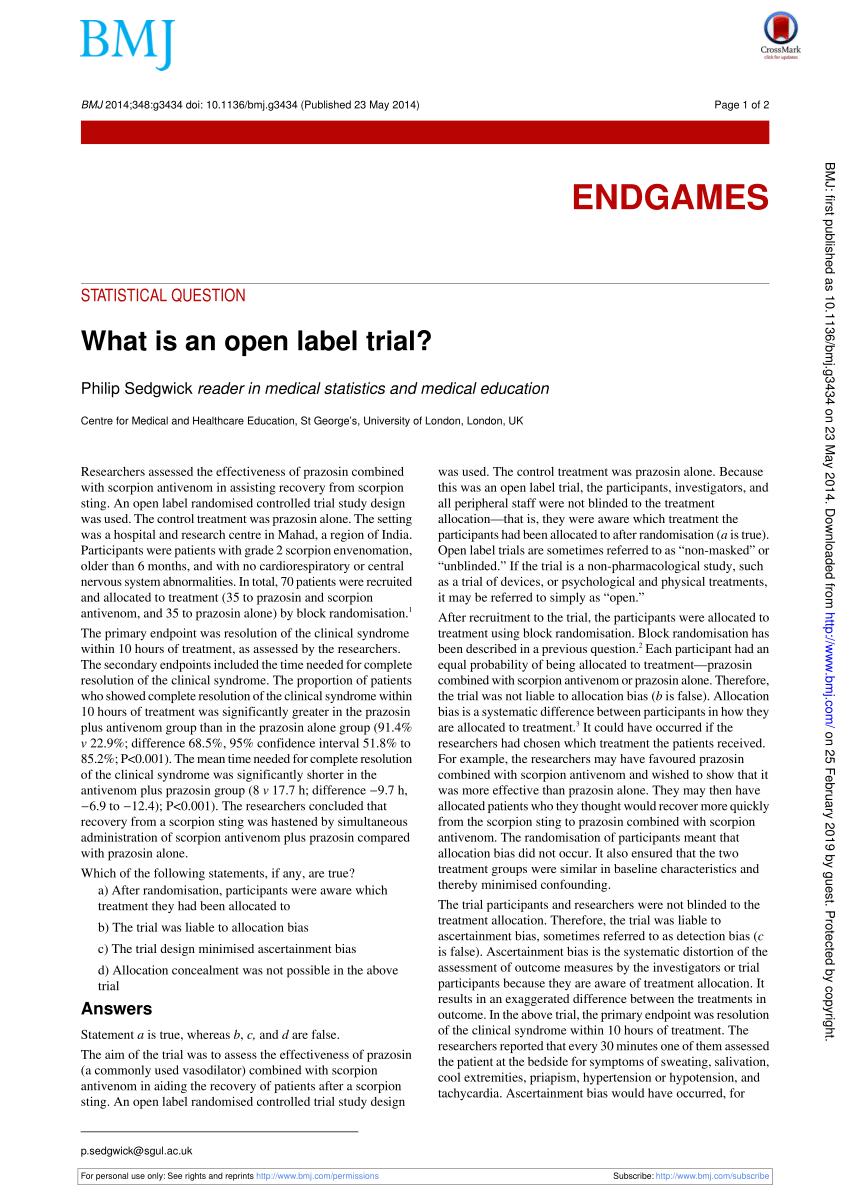
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. Effectiveness studies: advantages and disadvantages - Taylor & Francis An the context of evidence-based medicine, 1 randomized control-group trials (RCTs) are considered to be the decisive level of scientifically proven evidence as far as therapeutic aspects are concerned. 2 Placebocontrolled trials, especially for certain psychiatric indications, are ranked higher in terms of evidence than active control-group … Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely. PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub-
Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: •
Epidemiology and Clinical Research Design, Part 1: Study Types. Strengths and limitations of retrospective studies: The main advantages of studies using existing data are speed and cost. A retrospective analysis of an association between caffeine and necrotizing enterocolitis (NEC) can be conducted in a few months using a neonatal intensive care unit (NICU) database with minimal expense. ( 6) Strengths:
💋 Case study advantages and disadvantages. Case Study Method. 2022-11-03 A case study can focus on one person, a group, an organization, or an event. One of the biggest disadvantages is that they are often retrospective. This involves the use of either vertical or horizontal or both subunits of the focal organization as activity systems. Writing notes in diaries and journals about the entity is a good example of a ...
Open-Label Extension Studies | SpringerLink Open-label extension studies have been described as "case series [studies] of the survivors of the [double-blind] trials", [ 1, 2] inferring a range of deficiencies and potential biases in their design and interpretation, issues which will be discussed in this article.
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
External and internal validity of open label or double-blind ... - PubMed In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
5 Ups and Downs of Special Education Labels | Education.com Disadvantage #2: Special Education Services May Be Costly. Oftentimes the label comes too late. A preventative approach can help students with mild learning problems early on, which may reduce the number of students needing special education. This can also decrease money spent on special education and the stigma of a label.
PDF ACKNOWLEDGMENTS - National Pharmaceutical Council In practice, each research approach has advantages and disadvantages, and the research approach for a CER question should be selected based upon the specific features or characteristics of the study question. The purpose of this document is to provide brief descriptions of both experimental and nonexperimental study designs and methods that may
External and internal validity of open label or double‐blind trials in ... As a consequence, an intense discussion of the advantages and disadvantages of both designs is currently under way and interpretation of trial results is often focused on this matter. This review addresses the risks of bias for internal and external validity of open-label and double-blind anticoagulation trials to help to objectify this debate.
PDF Labeling and Disadvantages of Labeling - University of North Carolina ... scientists would be in raising money for cancer research if they had no name for it. The advantages of labeling can be summarized as follows: 1. Federal and local funding of special education programs are based on categories of disabilities. 2. Labeling enables professionals to communicate with one another because each categorical label
Reducing bias in open-label trials where blinded outcome assessment is ... This can be an issue in trials assessing surgical interventions, device trials, or other non-pharmacologic interventions, which are more difficult to blind than traditional drug trials [ 4 ]. Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations.
External and internal validity of open label or double ... - ResearchGate As a consequence, an intense discussion of the advantages and disadvantages of open-label or double-blind trials is currently under way and interpretation of trial results is often focused on this ...
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,...

















:max_bytes(150000):strip_icc()/Vertical-integration-7a31b884b9564a139c5ec2f7885ff3f0.jpg)

![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)
![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)




Post a Comment for "39 open-label study advantages and disadvantages"